 |
 |
|
 |
Search | Help | MolPaint | Roadmap | Credits | Feedback |
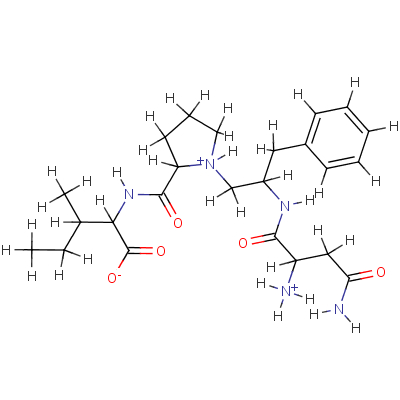 |
|
| download 2D Mol File
| download 3D Mol File
Potential Energy Epot(MMFF94)=74.2089 kcal/mol |
NCID-ZINC05882990 |
MMsINC code: MMs02509272 |
|
Type: Ionized Formula: C24H38N5O5+
|
 |
|
| download 2D Mol File
| download 3D Mol File
Potential Energy Epot(MMFF94)=74.2089 kcal/mol |
| Physical Properties | ||||||
| Molecular Weight: 476.598 g/mol | logS: -3.52064 | SlogP: -3.47213 | Reactive groups: 0 | |||
| Topological Properties | ||||||
| Globularity: 0.104198 | Sterimol/B1: 2.98915 | Sterimol/B2: 5.00417 | Sterimol/B3: 6.1624 | |||
| Sterimol/B4: 8.29477 | Sterimol/L: 18.9286 | |||||
| Surface and Volume Properties | ||||||
| Accessible surface: 770.608 | Positive charged surface: 534.318 | Negative charged surface: 236.29 | Volume: 472.875 | |||
| Hydrophobic surface: 495.48 | Hydrophilic surface: 275.128 | |||||
| Pharmacophoric Properties | ||||||
| Hydrogen bond donors: 3 | Hydrogen bond acceptors: 3 | Acid groups: 2 | Basic groups: 2 | |||
| Chiral centers: 5 | ||||||
| Drug- and Lead-like Properties | ||||||
| Lipinski's drug-like rule: 1 | Violations of Lipinski's rule: 0 | Oprea's lead like rule: 0 | ||||
|