|
 |
|
 |
Search | Help | MolPaint | Roadmap | Credits | Feedback |
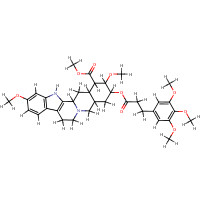
| SMILES: | O(C)C1C(C2C(CC1OC(=O)CCc1cc(OC)c(OC)c(OC)c1)C[NH+]1C(C2)c2[n H]c3cc(OC)ccc3c2CC1)C(OC)=O |
| InChI: | InChI=1/C35H44N2O9/c1-40-21-8-9-22-23-11-12-37-18-20-15-29(46-30(38)10-7-19-13-27(41-2)33(43-4)28(14-19)42-3)34(44-5)31(35(39)45-6)24(20)17-26(37)32(23)36-25(22)16-21/h8-9,13-14,16,20,24,26,29,31,34,36H,7,10-12,15,17-18H2,1-6H3/p+1/t20-,24-,26-,29+,31-,34+/m1/s1 |
 |
|
| download 2D Mol File
| download 3D Mol File
Potential Energy Epot(MMFF94)=131.941 kcal/mol |
MOE's Descriptors
| Physical Properties | ||||||
| Molecular Weight: 637.75 g/mol | logS: -5.34078 | SlogP: 3.16844 | Reactive groups: 1 | |||
| Topological Properties | ||||||
| Globularity: 0.110415 | Sterimol/B1: 3.43652 | Sterimol/B2: 4.07007 | Sterimol/B3: 7.81547 | |||
| Sterimol/B4: 10.7441 | Sterimol/L: 24.1489 | |||||
| Surface and Volume Properties | ||||||
| Accessible surface: 1012.21 | Positive charged surface: 842.643 | Negative charged surface: 163.341 | Volume: 614.375 | |||
| Hydrophobic surface: 904.319 | Hydrophilic surface: 107.891 | |||||
| Pharmacophoric Properties | ||||||
| Hydrogen bond donors: 1 | Hydrogen bond acceptors: 7 | Acid groups: 0 | Basic groups: 1 | |||
| Chiral centers: 6 | ||||||
| Drug- and Lead-like Properties | ||||||
| Lipinski's drug-like rule: 0 | Violations of Lipinski's rule: 2 | Oprea's lead like rule: 0 | ||||
|