|
 |
|
 |
Search | Help | MolPaint | Roadmap | Credits | Feedback |
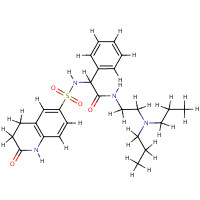
| SMILES: | S(=O)(=O)(NC(C(=O)NCC[NH+](CCC)CCC)c1ccccc1)c1cc2CCC(=O)Nc2c c1 |
| InChI: | InChI=1/C25H34N4O4S/c1-3-15-29(16-4-2)17-14-26-25(31)24(19-8-6-5-7-9-19)28-34(32,33)21-11-12-22-20(18-21)10-13-23(30)27-22/h5-9,11-12,18,24,28H,3-4,10,13-17H2,1-2H3,(H,26,31)(H,27,30)/p+1/t24-/m1/s1 |
 |
|
| download 2D Mol File
| download 3D Mol File
Potential Energy Epot(MMFF94)=40.4252 kcal/mol |
MOE's Descriptors
| Physical Properties | ||||||
| Molecular Weight: 487.645 g/mol | logS: -4.36267 | SlogP: 1.50747 | Reactive groups: 0 | |||
| Topological Properties | ||||||
| Globularity: 0.0712205 | Sterimol/B1: 3.75644 | Sterimol/B2: 3.90435 | Sterimol/B3: 5.05195 | |||
| Sterimol/B4: 8.69874 | Sterimol/L: 19.8715 | |||||
| Surface and Volume Properties | ||||||
| Accessible surface: 782.845 | Positive charged surface: 530.084 | Negative charged surface: 252.76 | Volume: 476.5 | |||
| Hydrophobic surface: 540.13 | Hydrophilic surface: 242.715 | |||||
| Pharmacophoric Properties | ||||||
| Hydrogen bond donors: 3 | Hydrogen bond acceptors: 4 | Acid groups: 0 | Basic groups: 1 | |||
| Chiral centers: 1 | ||||||
| Drug- and Lead-like Properties | ||||||
| Lipinski's drug-like rule: 1 | Violations of Lipinski's rule: 0 | Oprea's lead like rule: 0 | ||||
|