|
 |
|
 |
Search | Help | MolPaint | Roadmap | Credits | Feedback |
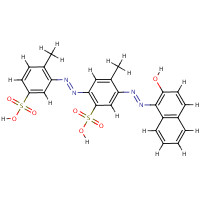
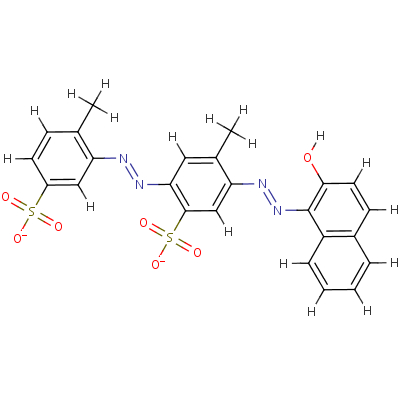
| SMILES: | S(=O)(=O)([O-])c1cc(N=Nc2c3c(ccc2O)cccc3)c(cc1N=Nc1cc(S(=O)( =O)[O-])ccc1C)C |
| InChI: | InChI=1/C24H20N4O7S2/c1-14-7-9-17(36(30,31)32)12-19(14)25-27-21-11-15(2)20(13-23(21)37(33,34)35)26-28-24-18-6-4-3-5-16(18)8-10-22(24)29/h3-13,29H,1-2H3,(H,30,31,32)(H,33,34,35)/p-2/b27-25+,28-26+ |
 |
|
| download 2D Mol File
| download 3D Mol File
Potential Energy Epot(MMFF94)=102.339 kcal/mol |
MOE's Descriptors
| Physical Properties | ||||||
| Molecular Weight: 538.561 g/mol | logS: -7.25053 | SlogP: 5.80124 | Reactive groups: 0 | |||
| Topological Properties | ||||||
| Globularity: 0.0727685 | Sterimol/B1: 3.54091 | Sterimol/B2: 3.80015 | Sterimol/B3: 7.15205 | |||
| Sterimol/B4: 7.45558 | Sterimol/L: 19.7587 | |||||
| Surface and Volume Properties | ||||||
| Accessible surface: 759.291 | Positive charged surface: 303.267 | Negative charged surface: 446.419 | Volume: 443.625 | |||
| Hydrophobic surface: 524.804 | Hydrophilic surface: 234.487 | |||||
| Pharmacophoric Properties | ||||||
| Hydrogen bond donors: 1 | Hydrogen bond acceptors: 5 | Acid groups: 6 | Basic groups: 0 | |||
| Chiral centers: 0 | ||||||
| Drug- and Lead-like Properties | ||||||
| Lipinski's drug-like rule: 0 | Violations of Lipinski's rule: 3 | Oprea's lead like rule: 0 | ||||
|